PROCESS SYSTEM
Automatic control
High performance material selection
High efficiency and low energy consumption
High performance equipment to keep system long-term operation
Automatic control
High performance material selection
High efficiency and low energy consumption
High performance equipment to keep system long-term operation
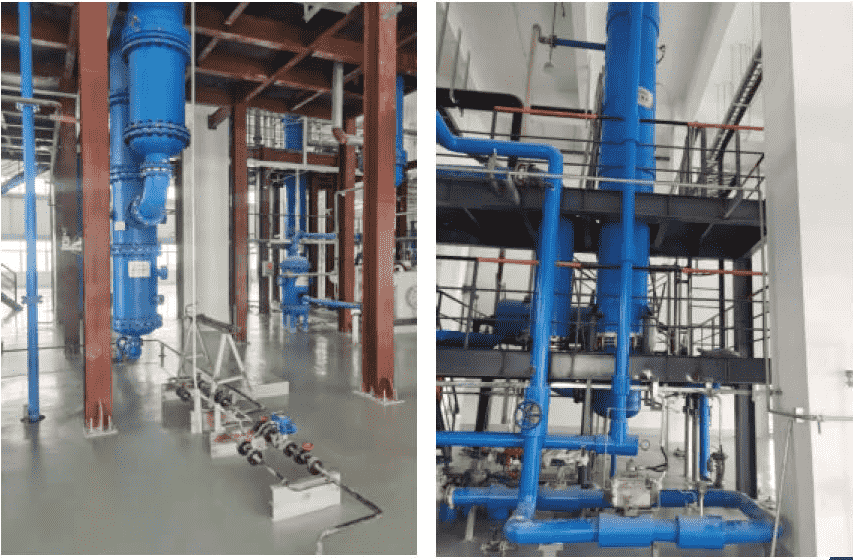
Under absolute pressure condition of 3-10 kPa, any concentration sulfuric acid becomes to sulfuric acidwith a concentration below 96 wt.% via horizontal heating and vaporization system or vertical compulsorcirculating heating and vaporization system using saturated steam or conduction oil as heating media.For large quantity, usually, the concentration system is divided into two-step or multi-step processes inorder to reach the demands of production concentrations.The SiC heat exchangeris the mainheat-exchanging equipment, using double-effect heat transfer, to greatly reduce the investment and runningcost.



For different process requirements and cost requirements to design different HCl drying system,the lowest water content in HCI gas can be less than 10PPm.

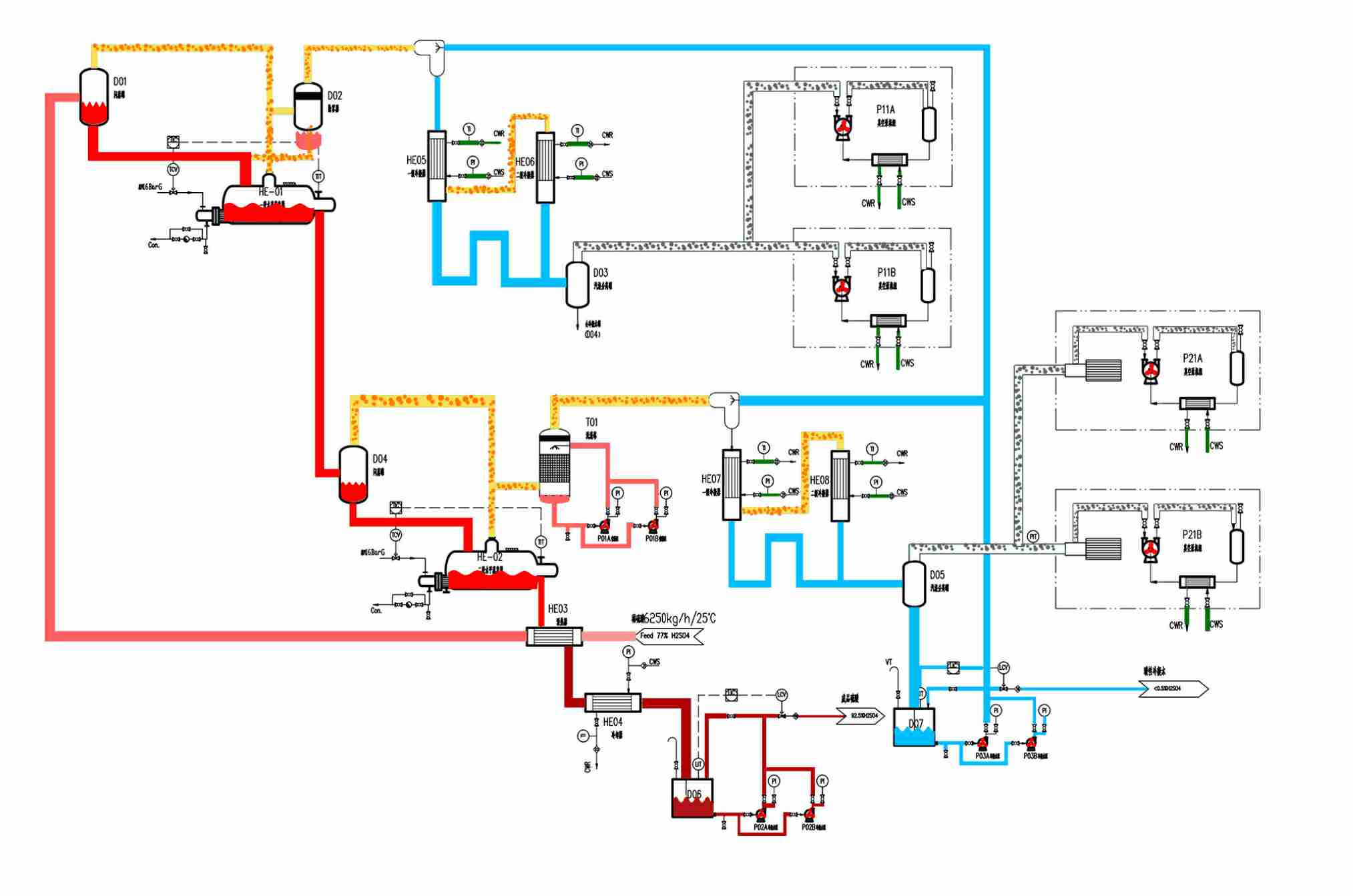
The liquid, which consists of any concentration HCL. acid heated by double-effect heat exchangers and hightemperature and high concentration CaCl2 solution in a certain proportion, flows in the top of the stripper. Hightemperature HCL. gas and water vapor from reboiler enter in the bottom of the stripper. The mixed liquid flowsdown, meantime, high temperature HCL gas and water vapor bubble up. Heat and mass transfer occursbetween them inside of the stripper, Under triphase azeotropic point, HCL gas is extracted sufficiently andcooled by a secondary condenser in the top of the stripper in order to gain dry and pressured HCL gas. Littlecondensate acid is back to the stripper to strippingDiluted CaCl2 solution flows into the CaCl2 evaporating tower, then, is heated by reboiler to remove most ofwater. Water vapor is condensed to water by condenser which contains less than 0.5% acid. The waste water iscollected by water pocket for the next usage.
Condensate CaCl2 solution is recycledby pressurized and mixed with HCL acid in a certain proportionflowing into the stripper.
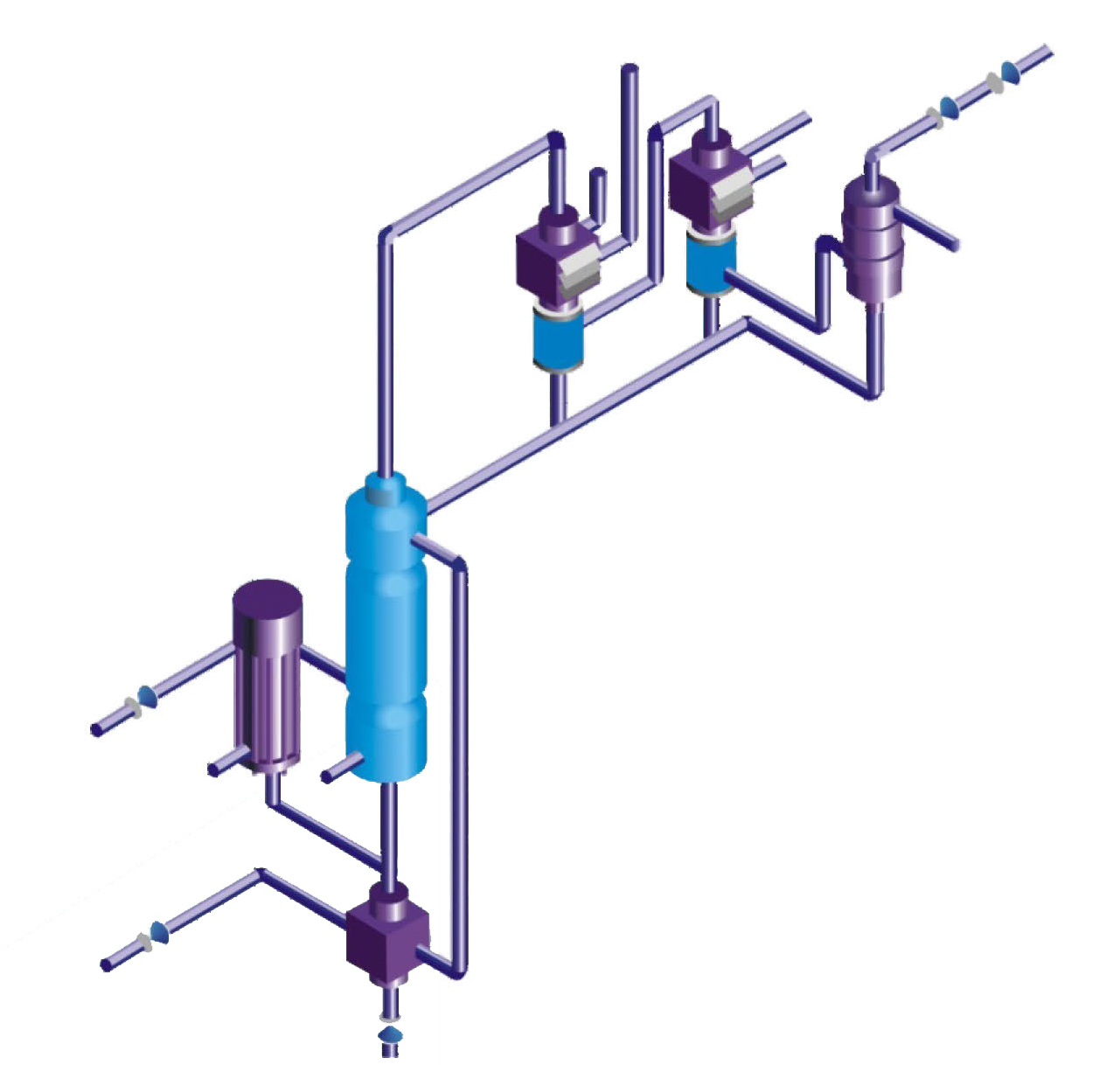


More than 15% dilute phosphoric acid is heated and evaporated with the heated phosphorioacid inthe silicon carbide faling film evaporator in the circulating circuit, and then flash evaporated in the ;separator.A large amount of phosphoric acid is heated through falling film evaporator by circulating pump.The evaporated sour water goes throuah the demister to remove P205 droplets, The gas phase is collectedby condensation, and the non condensable gas is discharged into the atmosphere byvacuum pump.By the way of indirect heating of silicon carbide falling film evaporator, the concentration waseffectively reduced to (85 ~105%). lt not only solves the problem of corrosion but alstso avoids scaling.